Evaluation of Certain Veterinary Drug Residues in Food: Seventy-Fifth Report of the Joint FAO/WHO Expert Committee on Food Addit | 生病了怎麼辦 - 2024年7月

Evaluation of Certain Veterinary Drug Residues in Food: Seventy-Fifth Report of the Joint FAO/WHO Expert Committee on Food Addit
This report represents the conclusions of a Joint FAO/WHO Expert Committee convened to evaluate the safety of residues of certain veterinary drugs in food and to recommend maximum levels for such residues in food.The first part of the report considers general principles regarding the evaluation of veterinary drugs within the terms of reference of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), including comments on documents under elaboration for the Codex Committee on Residues of Veterinary Drugs in Foods (CCRVDF), information on registration/approval status of veterinary drugs, extrapolation of maximum residue limits (MRLs), dietary exposure assessment methodologies, the decision-tree approach to the evaluation of residues of veterinary drugs and guidance for JECFA experts.Summaries follow of the Committee's evaluations of toxicological and residue data on a variety of veterinary drugs: two antimicrobial agents (amoxicillin, apramycin), an antiparasitic agent (derquantel), three anthelminthics (ivermectin, monepantel, triclabendazole) and two antimicrobial agents and production aids (monensin and narasin). Annexed to the report is a summary of the Committee's recommendations on these drugs, including acceptable daily intakes (ADIs) and proposed MRLs.
 大字版 3D圖解 穴道淋巴按摩 按...
大字版 3D圖解 穴道淋巴按摩 按... 遇見生機:跟著生機飲食專家王明勇一...
遇見生機:跟著生機飲食專家王明勇一...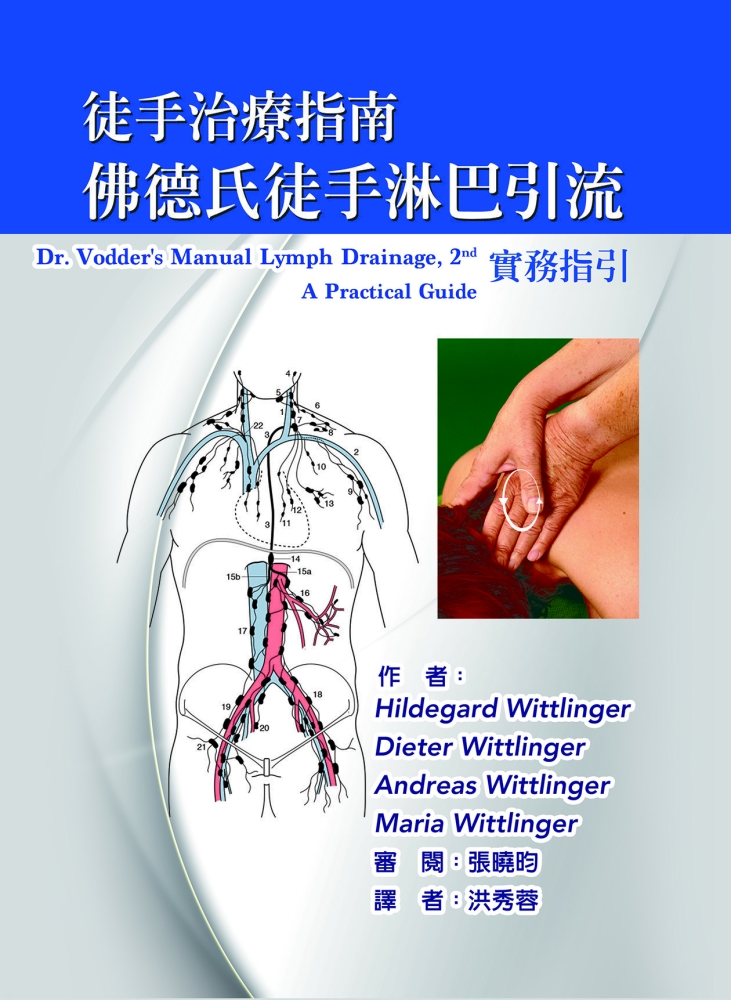 徒手治療指南 佛德氏徒手淋巴引流:...
徒手治療指南 佛德氏徒手淋巴引流:... 淋巴排毒全療法【白金完整版】: 五...
淋巴排毒全療法【白金完整版】: 五... 一本讀通 血癌:解答淋巴癌、白血病...
一本讀通 血癌:解答淋巴癌、白血病... 神奇淋巴循環瘦身術:每天5分鐘舒緩...
神奇淋巴循環瘦身術:每天5分鐘舒緩... 淋巴排毒健身操 BOOK2進階版(...
淋巴排毒健身操 BOOK2進階版(... 全彩圖解內臟淋巴按摩:掌握5大淋巴...
全彩圖解內臟淋巴按摩:掌握5大淋巴... 穴道指壓&淋巴按摩
穴道指壓&淋巴按摩 OUT老廢物!日本熱門健康節目大推...
OUT老廢物!日本熱門健康節目大推...